

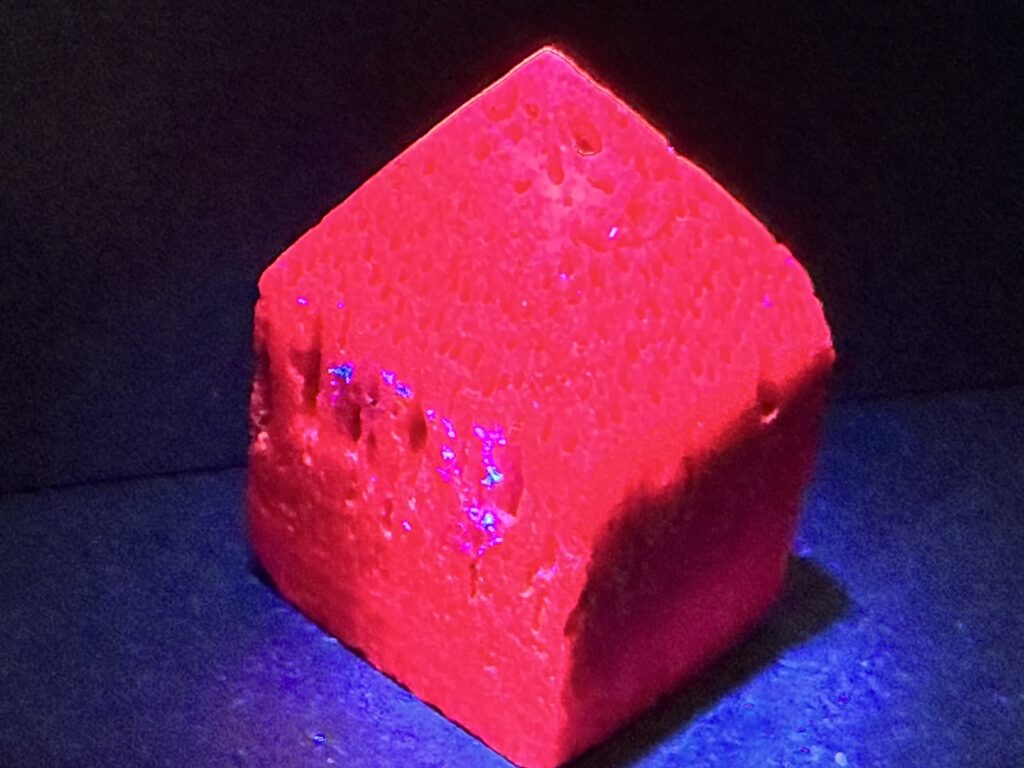
Ruby is one of the four precious gemstones (Ruby, Sapphire, Diamond, and Emerald) and is the red variety of corundum. While various red shades of the mineral corundum are considered a ruby, not all rubies are dark when under ultraviolet light. Some light up beautifully as the tower in my photo above. Typically, chromioum is the trace element that gives a ruby it’s red color–ranging from an orangy-red to a purplish- red. The more chromim a ruby has, the redder it is.
Although chromium can cause fluorescence, rubies that do not have iron have a better chance at fluorescing than those with iron. Rubies are formed in many places throughout the world, however the locations for some of the best quality gems are those where the gem is formed in marble versus basalt.
The factor that contributes to the fluorescence of a ruby is the lack of iron. The Gemological Institute of America notes that higher iron content in the chemical makeup of a ruby can mask its fluorescence. The fluorescence is what gives rubies their glow and this is one of the key things gemologists will test for when identifying a ruby.
It’s important to note that not all rubies that don’t fluoresce are fake rubies as some sites suggest. I have seen claims that only rubies that fluorces are real and others that don’t are fake. This simply isn’t true. Rubies are a gem variety of the mineral corundum which is a rock-forming mineral and a crystalline from of aluminum oxide. It has two aluminum atoms and three oxygen atoms in a tightly packed hexagonal structure.
The rubies with iron don’t fluoresce but are still rubies.
